Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Review Article(ISSN: 2637-4706) 
Formulation Development and Evaluation of Fast Disintegrating Tablets- A Review Volume 4 - Issue 1
Raghavendra Kumar Gunda*, JN Suresh Kumar, B Yamini, B Naveena, L Sravani, SK Mansur Ali and Ch Priyanaka
- Department of Pharmaceutics, Narasaraopeta Institute of Pharmaceutical Sciences, India
Received: November 17, 2022; Published:November 30, 2022
Corresponding author:Raghavendra Kumar Gunda, Associate Professor, Department of Pharmaceutics, Narasaraopeta Institute of Pharmaceutical Sciences, India
DOI: 10.32474/DDIPIJ.2022.04.000180
Abstract
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
The drug delivery industry is currently experiencing intense competition and quick evolution due to an ever-growing demand. One such novel and distinctive drug delivery method that is quickly gaining popularity in the realm of rapid dissolving technology is the fast-dissolving tablet (FDT). Because a large variety of medications are supplied through this route, oral administration is the fastest and safest method of drug delivery. Since the ancient decade, oral administration has received significantly greater attention for the treatment or management of disorders. Mouth dissolving tablets (MDTs), a novel idea in oral delivery, are now widely used. Without the use of water, fast-dissolving tablets swiftly disintegrate or dissolve in the mouth. Some tablets are truly fast-dissolving tablets because they are made to dissolve in saliva amazingly quickly-within a few seconds.
Keywords:Mouth dissolving tablet; super disintegrants; oral route; paediatric; geriatric
Introduction
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
A fast-dissolving tablet is a solid dosage form that can quickly dissolve in the mouth and produce a solution or suspension without the need for water to be administered. Up to 50–60% of all dosage forms are administered by oral methods, which are well accepted. Solid dosage forms are widely used due to their patient compliance, convenience of administration, precise dosing, self-medication, and pain relief. In the late 1990s, the first fasting dissolving drug delivery systems were created as an alternative to traditional dosage forms for paediatric and geriatric patients. These pills are made to dissolve or disintegrate quickly in the saliva, usually in less than 60 seconds [1-3]. Through the mouth and GIT mucous membranes, the active components in this are absorbed and enter the bloodstream. Academics and industry are increasingly recognising the benefits of mouth-dispersing dose formulations. Due to several drawbacks of rapid dissolving tablets, such as their sometimes difficult to carry, store, and handle physical solid form, or their potential to leave an unpleasant taste or grittiness in the mouth if improperly manufactured. Fast-dissolving oral films are a novel technology created to protect the dose form and solve these issues.
Requirements of Fast Dissolving Drug Delivery System: [4]
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
The tablets must adhere to the following standards
a) It should melt or disintegrate in the mouth in a couple of
seconds without the need for water.
b) Work well with flavour muffling.
c) Be transportable without posing a fragility risk.
d) Feel good in the mouth.
e) No chewing is required.
f) Low sensitivity to environmental factors like humidity
and temperature.
A Fast-Dissolving Drug Delivery System’s Key Characteristics
a) The dosage form does not require water to be swallowed,
which is a very practical aspect for people who are travelling
and may not have easy access to water.
b) Ease of administration for patients who are unable to
swallow (Dysphagia), such as stroke sufferers or the elderly.
c) Especially in paediatric patients, the good mouth feel
feature helps to modify the perception of medication as an
unpleasant tablet.
d) A higher bioavailability as a result of the tablets’
quick dissolving and disintegration, especially in the case of
hydrophobic and insoluble medicines.
e) The drug will dissolve and absorb quickly, leading to an
immediate start of action.
Mechanism of tablets with rapid dissolution [5,6]:
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
In order to obtain the tablets’ quick-dissolving qualities, tablets possess must possess following; For the tablet to instantly dissolve and disintegrate, water must swiftly enter the matrix of the tablet. The formulation of the tablet includes a suitable disintegrant or highly water-soluble excipients. These are a few less-discussed methods for medication suspension from broken tablets.
The mechanisms are:
a) High swell ability of disintegrates
b) Chemical reaction.
c) Capillary/ Wicking action (Cavernous).
Advantages of Quickly Dissolved Dosage Forms
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
a) Helpful in situations where an ultra-rapid beginning of
action is necessary, such as motion sickness and allergic attack
coughing spells.
b) Administered anytime, anywhere, and without water.
c) Intervention with rapid pharmacological therapy.
d) Quick start-up time and potential for increased
bioavailability.
e) Patient compliance has improved.
f) Conventional machinery for manufacturing.
g) As well as the typical oral solid dose form, good chemical
stability.
h) Longer-lasting stability because the medicine stays in
solid dosage form until it is taken.
i) Improved Safety.
Limitations of Orally Disintegrating Tablets
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
a) It is challenging to create tablets that dissolve in the
tongue for medications with relatively higher doses [7,8].
b) If the tablets are not made properly, they may leave a bad
taste and/or grittiness in the mouth6.
c) The mechanical strength of the tablets is typically
insufficient. As a result, careful handling is necessary.
Techniques for Making of Fast-Dissolving Tablets
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
a) Lyophilisation
b) Tablet moulding
c) Direct compression
d) Cotton candy process
e) Spray drying
f) Sublimation
g) Mass extrusion
h) Nanonization
i) Fast dissolving films
Lyophilisation
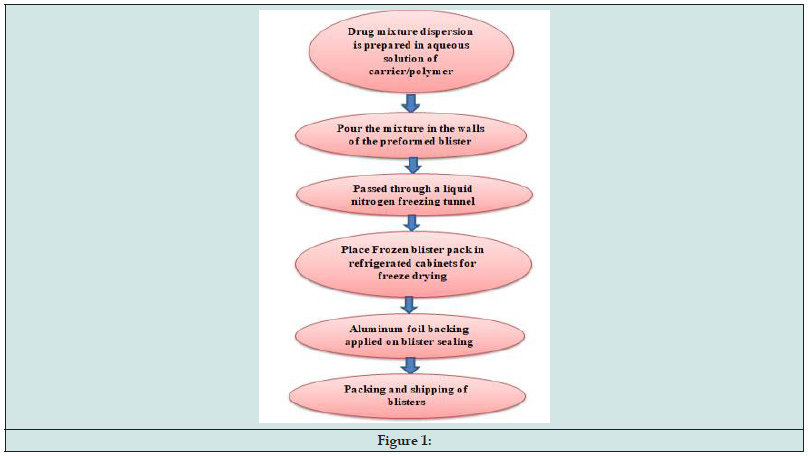
It is a procedure that involves removing the solvent from a medication suspension or solution that has been frozen and contains additives that form structures. This method has shown to boost bioavailability and improve absorption. The addition of chemicals and freeze-drying of the medication creates a glossy, amorphous structure that results in a very porous and light product. When placed on the tongue, the resulting tablet quickly disintegrates and dissolves, and the freeze-dried component dissolves right away to release the medication. Here is a typical process used in the production of FDT made with this technology [9].
Advantages
a) Greater control over fill weight.
b) One can preserve sterility.
c) Water removal at a low temperature.
d) Appropriate for aseptic operation.
Disadvantages
a) It is costly and time-consuming.
b) Poor stability when under stress.
c) Due to their fragility, conventional packaging is
inappropriate for these products (Figure 1).
Tablet Moulding
The very porous structure of tablets created by the moulding process leads to a rapid rate of dissolution and disintegration. This procedure entails moistening, dissolving, or dispersing the medications with a solvent before compressing the moist mixture into tablets with a lower pressure—always lowers than the compression used for traditional tablet manufacturing. Moulded tablets’ low mechanical strength causes handling-related erosion and fracture [10,11]. There are two different types of moulding processes: solvent method and heat method.
a) Solvent Method: The solvent approach produces fewer
compact tablets with a porous structure that speeds up dissolve
than compressed tablets do.
b) Heat Method: The molten mass containing the medication
is set via a method called heat moulding. In this method, the pills
are made using a mould, agar solution, and blister packaging
(Figure 2).
Advantages
a) The most portable and lightweight dose form.
b) Simple to administrate
c) It is simple to use.
d) More stable.
Direct compression
The simplest and most economical method of producing tablets is direct compression. Due to the availability of better excipients, specifically super disintegrates and sugar-based excipients, this approach can now be used for the formulation of FDT. Super disintegrants: Super disintegrants are mostly added to direct compression to impact the rate of disintegration and subsequently the dissolution. Utilizing sodium starch glycollate, croscaramellose sodium and crospovidone as well as the super disintegrants allows the FDT to dissolve quickly. Sugar based excipients: Another method for producing tablets that dissolve quickly through direct compression is this one. Use of sugar-based excipients, in particular diluents like dextrose, fructose, maltose, mannitol, sorbitol, starch hydrolysate, polydextrose, and xylitol, which exhibit high aqueous solubility and sweetness and hence impart flavour masking property and a good mouth feel [12].
Advantages
a) Because fewer unit operations are needed in
manufacturing processes, batch to batch variations are minimal.
b) Consistency in particle size.
c) Greater ageing stability of the tablet.
d) Problems with chemical stability for API and excipients
would be prevented.
Cotton Candy Process
This method gets its name from the floss-like crystal structures it creates using a special spinning mechanism, which resemble cotton candy. Larger pharmacological doses can be accommodated by this method, which also provides increased mechanical strength. However, the utilisation of this technique is constrained by high process temperatures.
Spray Drying
By spraying the feed into a hot drying medium, feed can be converted from a fluid condition to a dried particle form. In this approach, mannitol serves as the bulking agent, whereas crosscarmellose, sodium starch glycolate, and crospovidone are utilised as super disintegrants. The tablets made from spray-dried powder that contain a super disintegrant, a bulking agent, an acidic component (citric acid), and/or an alkaline ingredient (such as sodium bicarbonate), have been reported to dissolve in aqueous medium in under 20 seconds. This compacted powder that had undergone spray drying performed better and disintegrated more quickly.
Advantages
a) Process that is continuous and simple to manage.
b) Since the materials are dried in a single process without
handling, labour costs are cheap.
c) The particle size and shape of the dry power will be
consistent.
Disadvantages
a) Spray dryers are unable to dry solid objects.
b) Due to its poor thermal efficiency, a significant amount of
heat is lost during use.
c) It’s challenging to clean after usage.
Spray drying equipment is expensive to install and cumbersome.
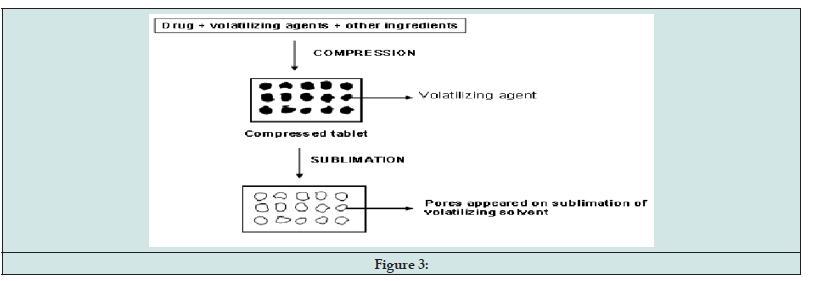
For fast-dissolving tablets, the matrix of the tablet must have a porous structure in order to dissolve quickly. The process of sublimation is used to create porous mixtures by including volatile components.Benzoic acid, ammonium bicarbonate, ammonium carbonate, camphor, naphthalene, urea, and urethane are examples of substances that can be compacted into a tablet along with other excipients. This flammable substance is subsequently eliminated through sublimation, leaving behind a very porous matrix (Figure 3).
Advantages
a) The primary benefit of sublimation is its usage in the
purifying process.
b) No solvents are employed.
c) Loss is the absolute minimum amount of product.
Disadvantages
Under heat, a non-sublimable substance may break down.
Mass Extrusion
Using a solvent mixture of water-soluble methanol and polyethylene glycol, the active blend is softened in this method. The softened mass is then ejected through a syringe to produce a cylinder product, which is then cut into even segments using a hot blade to create tablets. The dried cylinder can also be used to coat bitter medicine pellets in order to hide their flavour [13] (Figure 4).
Nanonization
By adopting a specialised wet-milling procedure, a drug’s particle size is reduced to nano size using a recently developed nano melt technology. This method is particularly beneficial for poorly water-soluble medicines. Other benefits of this technology include quick dissolving of nanoparticles, which increases absorption and, as a result, improved bioavailability and dose reduction, a costeffective manufacturing method, traditional packaging, and a wide range of doses and remarkable durability [14,15].
Techniques for Evaluating Fast Dissolving Tablets
To assess the quality of the tablet, it is critical to consider how the medications are produced.
Wetting time [16]
The contact angle and dosage form wetting time are connected. A straightforward process can be used to measure the pills’ wetting time. A petridish with a 10 cm diameter is filled with five round tissue papers. Petridish is mixed with ten millimetres of the watersoluble dye Eosin. The tissue paper is gently placed on top of the tablet. Wetting time is the time needed for water to reach the tablet’s upper surface.
Hardness [17]
Utilizing hardness testers such as those made by Pfizer and Monsanto, among others, the tablets’ hardness was tested. The amount of force needed to break the tablets is proportional to how hard they are (kg/cm2). The results must match the required value. It is the amount of force needed to break a tablet by compression in the radial direction. This is a crucial consideration when formulating mouth dissolve tablets since too much crushing strength drastically shortens the time it takes for the tablet to dissolve.
Friability [18]
A Roche friabilator was used to assess the tablets’ friability.
In a plastic chamber that rotates at 25 revolutions per minute,
this gadget shocks and abrades the tablets simultaneously while
dropping them from a height of 6 inches with each revolution. The
friabilator received a pre-weighed sample of tablets and was rotated
100 times. The tablets were reweighed after being de-dusted using
a delicate muslin cloth.
The friability (%) is given by the formula:
Disintegration Test
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
For FDTs, the disintegration test is also frequently used. The USP disintegration test instrument is used to calculate disintegration time. Each batch of six tablets includes a disintegration test. The disintegration test is conducted in 900 ml of pH 6.8 simulated saliva fluid at a rate of 30 2 cycles per minute with a temperature at 37±2ºC [19,20].
Drug Content Uniformity
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
Twenty tablets were measured, taken, then crushed to form powder in a mortar. A quantity of powder weighing equivalent to 100 mg of tablet powder was taken in 100 ml volumetric flask and was dissolved in suitable solvent as specified in individual monograph. suitable aliquots were prepared by filtration using membrane filter 0.45μm and then the solutions absorbance was measured at Wavelength Maxima (λMax, nm) [21].
Dissolution Test
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
The process used to perform dissolution testing for FDTs is analogous to and nearly equivalent to that used for regular tablets. The dissolution testing of drugs that have been taste-masked is also suitable for the USP 2 Paddle equipment at 50-100 rpm. To enhance the test’s usefulness, the media for the taste-masked medication should be the same as the final product. Higher paddle speeds can avoid the production of large, over-one-gram tablets that include relatively dense particles in the dissolution vessel10. Since the formulation is intended to have a pleasant taste and feel, the excipient to drug ratio may be larger, which reduces the signal of the drug to background (excipient) in the UV spectrophotometric approach. These two circumstances raise the recommended stirring speed to 25–75 rpm [22,23].
Moisture Uptake Studies [24,25-28]
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
For FDT, moisture uptake tests should be carried out to get insight into the formulation’s stability. Ten tablets of each formulation were stored for 24 hours at 37°C in a desiccators over calcium chloride. The tablets were then weighed and kept at room temperature for two weeks while being exposed to 75% RH. Saturated sodium chloride solution was left in the bottom of the desiccators for three days to provide the necessary humidity. To evaluate the moisture uptake caused by various excipients, one tablet was preserved as the control (without super disintegrating agents). Tablets were weighed, and the weight gain as a percentage was noted.
Conclusion
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
Fast-dissolving tablets primary goals are to increase patient compliance and convenience. They are considered to be a very good substitute for administering medications to elderly and paediatric patients. one can take these pills with or without water.
References
-
<
- Abstract
- Introduction
- Requirements of Fast Dissolving Drug Delivery System: [4]
- Mechanism of tablets with rapid dissolution [5,6]:
- Advantages of Quickly Dissolved Dosage Forms
- Limitations of Orally Disintegrating Tablets
- Techniques for Making of Fast-Dissolving Tablets
- Disintegration Test
- Drug Content Uniformity
- Dissolution Test
- Moisture Uptake Studies [24,25-28]
- Conclusion
- References
- Kushagra Khanna, Gauravi Xavier, Suresh Kumar Joshi, Aashish Patel, Sakshum Khanna et al. (2016) Fast dissolving tablets, International journal of pharmaceutical research and allied sciences 5(2): 311-322.
- Ashish Masih, Amar Kumar, Ajay Kumar Tiwari, Shivam Singh (2017) Fast dissolving tablets: An overview. International journal of current pharmaceutical research 9(2): 8-18.
- Nautiyal U, Singh S, Singh R, Gopal, Kakar S (2014) Fast dissolving tablets as a novel boon: A review. J Pharm Chem Biol Sci 2(1): 5-26.
- Debjit Bhowmik, Krishnakanth, Pankaj, R Margret Chandira (2009) Fast dissolving tablets-An Overview. Journal of chemical and pharmaceutical research 1(1): 163-177.
- Dinesh Kumar Pandurangan,Tejaswi Vuyyuru, Dhatrija Kollipara (2012) Fast dissolving tablets: An overview. International Journal of Research in Pharmaceutical Sciences 3(2): 348-355.
- Shailendra Kumar Sah, Ashutosh Badola (2017) Mouth dissolving tablet: A better approach to drug delivery. International journal of research in pharmacy & chemistry 7(1): 20-29.
- Ashok Kumar, Varun Bhushan, Manjeet Singh, Arti Chauhan (2011) A review on evaluation and formulation of fast dissolving tablets. International journal of drug research and technology 1(1): 8-16.
- Raghavendra Kumar Gunda, JN Suresh Kumar (2018) Formulation Development and Evaluation of Amisulpride Fast Dissolving Tablets. FABAD J Pharm Sci 43(2): 105-115.
- Gunda Raghavendra Kumar, Suresh Kumar J N, V Satyanarayana (2016) Formulation Development and Evaluation of Clopidogrel Fast Dissolving Tablets. Iran J Pharm Sci 12 (2): 61-74.
- Deepak Heer, Geeta Aggarwal, Hari Kumar (2013) Recent trends of Fast dissolving tablets: An overview of formulation technology. Pharmacophore 4(1): 1-9.
- Raghavendra Kumar Gunda, Suresh Kumar JN (2017) Formulation Development and Evaluation of Moxifloxacin Fast Dissolving Tablets. Pharm Methods 8(2): 160-167.
- Bandari S, Mittapalli RK, Ramesh Gannu, Rao YM (2008) Orodispersible tablets: An overview. Asian Journal of Pharmaceutics 2(1): 2-11.
- Gunda RK, Manchineni PR, Sumanjali YS, Somepalli Veeranjaneyulu, Penumula Ramanamma, et al. (2020) Design formulation and characterization of ODT for Lamotrigine. J Anal Pharm Res 9(2): 60-66.
- Abdulraheman ZS, Patel MR, Patel KR (2014) A review on immediate release tablet. Int J Univers Pharm Bio Sci 3: 93-113.
- Shukla D, Chakraborty S, Singh S, Mishra B (2009) An overview of formulation of mouth dissolving tablets. Sci Pharm 77(2): 309-326.
- Md Nehal Siddiqui, Garima Garg, Pramod Kumar Sharma (2010) Fast dissolving tablets: Preparation, characterization and evaluation: An overview. International Journal of Pharmaceutical Sciences Review and Research 4(2): 87-96.
- Uma Shankar Mishra, Prajapathi SK, Bhardvaj PA (2014) Review article on Mouth Dissolving tablets. International journal of research in pharmacy research & review 3(6): 24-34.
- Mangesh Machhindranath Satpute, Nagesh Shivaji Tour (2013) Formulation and in-vitro evaluation of Fast dissolving tablets of metaprolol tartrate. Brazilian Journal of Pharmaceutical Sciences 49(4): 783-792.
- Ramakant Joshi, Navneet Garud, Wasim Akram (2019) Fast dissolving tablets: A review. International journal of pharmaceutical sciences and research 11(4): 1562-1570.
- Raghavendra Kumar Gunda, JN Suresh Kumar, V Satyanarayana , Swathi Batta, Ch Meher Harika (2016) Formulation Development and Evaluation of Carbamazepine Fast Dissolving Tablets. J Pharm Res 10(5): 216-25.
- K Gnanaprakash, K Mallikarjuna rao, KB Chandra Sekhar, C Madhusudhana Chetty, M Alagusundaram, et al. (2009) Formulation and Evaluation of fast dissolving tablets of Valdecoxib. International journal of Pharma Tech Research 1(4): 1387-1393.
- D. Rahane, Punith R Rachh (2018) A review on fast dissolving tablets. Journal of drug delivery and therapeutics 8(5): 50-55.
- Keshari A, Tripathi KP, Srivastava A, Vishwas R (2015) Formulation & evaluation of effervescent floating tablets of antidiabetic drug. Journal of Drug Delivery and Therapeutics 5(6): 43-55.
- Sagar Konapure, Prafulla Chaudhari, Rajesh Oswal, Trushal Chorage, RV Antre (2011) Mouth dissolving tablets-An innovative technology. International journal of applied biology and pharmaceutical technology 2(1): 496-503.
- Allen T (1997) In Particle size measurement, 5th ed. Chapmanand hall, London, UK pp. 49-187.
- Alvarez-Lorenzo C, Gomez Amoza JL, Marty nez Pacheco R, Souto C, A Concheiro (2000) Evaluation of loe-substituted hydroxypropyl cellulose as filler binders for direct compression. Int J Pharma 197(1-2): 107-116.
- Sunada H, Bi Y (2002) Preparation evaluation and optimisation of rapidly disintegrating tablets. Powder Technology 122(2-3): 188-198.
- Koizumi KI, Watanable Y, Morita K, Utoguchi N, Matsumoto M (1997) New method for preparing high porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a sublingual material. Int J Pharma 152: 127-131.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...